Brandon Talbot | Sales Representative for Cityscape Real Estate Brokerage, Brandon Talbot | Over 15 Years In Real Estate. A tRNA molecule is a single-stranded molecule that exhibits significant intracellular base pairing, giving it its characteristic three-dimensional shape. At first, this seems pretty weird: doesn't A base-pair with U, and G with C? For example, of the four proline residues of ribonuclease A, at least two are thought to be in the trans configuration in order to form a native structure, whereas one or both of the other residues may be accommodated in either the cis or trans configuration. Here, well take a closer look at ribosomes and tRNAs. The degradome has been shown to affect many crucial biochemical pathways. refold into a different and infectious three-dimensional shape that kills cells in the brain and nervous system. From my understanding, it won't apply to the start codon, AUG, because there is only one possible codon available. Wellnot always. = Some tRNAs can form base pairs with more than one codon. for the trans isomer (antiperiplanar conformation). Thus, RNA clearly does have the additional capacity to serve as genetic information. 1999-2023, Rice University. WebThese proteins are also called polypeptides. tRNAs move through these sites (from A to P to E) as they deliver amino acids during translation. Hi, where does the Amino Acid comes from? This effect is due to amidoimido tautomerization. Illustration of the molecules involved in protein translation. WebPeptide Bond. (Biology is full of surprises, isn't it?)  A. Trezza, O. Spiga, in Cancer-Leading Proteases, 2020. This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (CONH). A new tRNA (in this case, one bearing Phe) will bind to the newly exposed codon in the A site, and the process can then repeat. The chelating peptide, Z-Tyr-Leu-hydroxamate inhibits leishmanolysin in the high micromolar range (17M), which is much lower than the millimolar concentrations needed for inhibition with 1,10-phenanthroline [22]. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. Figure 276.1. The formation of the peptide bond consumes energy, which, in organisms, is derived from ATP. The linear sequence of amino acids is read from left to right, with the amino terminal on the left. Figure 4.3. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. The tRNA for phenylalanine has an anticodon of 3'-AA. However, the broad absorption peak of the peptide bond allows measurements at longer wavelengths, which can have many practical advantages in terms of instrumentation and measurement accuracy. This linkage is found along a peptide or protein chain. A generalized illustration of how mRNA and tRNA are used in protein synthesis within a cell.
A. Trezza, O. Spiga, in Cancer-Leading Proteases, 2020. This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (CONH). A new tRNA (in this case, one bearing Phe) will bind to the newly exposed codon in the A site, and the process can then repeat. The chelating peptide, Z-Tyr-Leu-hydroxamate inhibits leishmanolysin in the high micromolar range (17M), which is much lower than the millimolar concentrations needed for inhibition with 1,10-phenanthroline [22]. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. Figure 276.1. The formation of the peptide bond consumes energy, which, in organisms, is derived from ATP. The linear sequence of amino acids is read from left to right, with the amino terminal on the left. Figure 4.3. This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. The tRNA for phenylalanine has an anticodon of 3'-AA. However, the broad absorption peak of the peptide bond allows measurements at longer wavelengths, which can have many practical advantages in terms of instrumentation and measurement accuracy. This linkage is found along a peptide or protein chain. A generalized illustration of how mRNA and tRNA are used in protein synthesis within a cell.  requires that the partial double bond be broken, so that the activation energy is roughly 80kJ/mol (20kcal/mol). WebWhere do peptide bonds form in the ribosome? [11] This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization. {\displaystyle \omega =180^{\circ }} For the peptide bond, bond angles and bond lengths indicate that carbonnitrogen bonds have a significant amount of double-bond character, and that the C, O, N, and H atoms all lie in the same plane. Ramachandran recognized that steric collisions between atoms prevent some combination of and angles and, for the trans configuration, ranges of and angles fall into defined regions in a graph called the Ramachandran plot (Figure 13.6) [26]. The ribosome moves along the mRNA. Due to interference from solvents and components of biological buffers, absorbance at 214 and 220nm is often used as an alternative to measure proteins and peptides. L/F-transferase is the sole member of this class of enzyme found in {\displaystyle \omega =\pm 90^{\circ }} Sure, these days you can find anything you want online with just the click of a button. The side chains are able to extend out from the peptide chain and interact with each other or with other molecules. Used tRNA molecules exit the ribosome and collect another specific amino acid. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S ribosomal subunit. and you must attribute OpenStax. According to permitted , , and angles, preferred conformations of the main chain lead to recurrent structures in proteinsnamely, alpha helix, beta sheets, and turns. During the formation of this bond, there is a release of water (H 2 O) molecules. It carries the correct amino acid to the site of protein synthesis in the ribosome. The wavelength of absorption for a peptide bond is 190230nm,[12] which makes it particularly susceptible to UV radiation. After peptide bond formation, the first step of translocation is a movement of the 3 ends of the tRNA from A to P and P to E sites of the 50S subunit, independent of the stationary anticodon ends. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. = This book uses the Be sure of your position before leasing your property. This is the pathway followed in proteolysis and, more generally, in NO acyl exchange reactions such as those of inteins. N.V. Bhagavan, Chung-Eun Ha, in Essentials of Medical Biochemistry, 2011. Further, the fact that the peptide bond TyrLeu is often cleaved but not always at the same rate, and in glucagon not at all, clearly indicates the importance of the amino acids flanking the scissile bond. You are correct, this article deals with prokaryotic translation. The peptide bond specificity of leishmanolysin from L. major was investigated using synthetic peptide substrates (Figure 276.1); it appears to be defined essentially by the P subsites of the substrate. The designation for the angle of rotation of the -carbonnitrogen bond is , whereas that of the -carboncarbonyl carbon bond is . G.N. If DNA serves as the complete library of cellular information, mRNA serves as a photocopy of specific information needed at a particular point in time that serves as the instructions to make a protein. Show transcribed image text. Future plans, financial benefits and timing can be huge factors in approach. Rotation is possible around these single bonds. This creates a peptide bond between the C terminus of the growing polypeptide chain and the A site amino acid. Gas-phase free-electron transmission spectra (ETS) of Gly, Ala, Phe, Pro and Cys [269] show that short-lived anions are formed by temporary attachment into the * empty orbitals of the COOH group of those amino acids. This process Both subunits are made up of both ribosomal RNA and proteins. [2] In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. In the recent years, degradomics has experienced a remarkable growth, not only in terms of number of known proteases but also in terms of biological and pathological roles played by the degradome. The trans and cis configurations of peptide bonds involving proline. As we saw briefly in the introduction, molecules called transfer RNAs (tRNAs) bring amino acids to the ribosome. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Prions are created when normal proteins in the body ____. So, a tRNA is is L shaped in 3D and clover leaf shaped in 2D? Thus, almost all the progress in terms of number of known proteases has been through the inclusion of new protein families which were not previously known to display proteolytic activity (Perez-Silva et al., 2016). This effect is due to amidoimido tautomerization. Direct link to Priyanka's post ATP is used to bind the a, Posted 5 years ago. As an Amazon Associate we earn from qualifying purchases. WebWhereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3).However, X-ray data suggest that the trans form occurs more frequently in proteins then you must include on every digital page view the following attribution: Use the information below to generate a citation. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction.
requires that the partial double bond be broken, so that the activation energy is roughly 80kJ/mol (20kcal/mol). WebWhere do peptide bonds form in the ribosome? [11] This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization. {\displaystyle \omega =180^{\circ }} For the peptide bond, bond angles and bond lengths indicate that carbonnitrogen bonds have a significant amount of double-bond character, and that the C, O, N, and H atoms all lie in the same plane. Ramachandran recognized that steric collisions between atoms prevent some combination of and angles and, for the trans configuration, ranges of and angles fall into defined regions in a graph called the Ramachandran plot (Figure 13.6) [26]. The ribosome moves along the mRNA. Due to interference from solvents and components of biological buffers, absorbance at 214 and 220nm is often used as an alternative to measure proteins and peptides. L/F-transferase is the sole member of this class of enzyme found in {\displaystyle \omega =\pm 90^{\circ }} Sure, these days you can find anything you want online with just the click of a button. The side chains are able to extend out from the peptide chain and interact with each other or with other molecules. Used tRNA molecules exit the ribosome and collect another specific amino acid. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S ribosomal subunit. and you must attribute OpenStax. According to permitted , , and angles, preferred conformations of the main chain lead to recurrent structures in proteinsnamely, alpha helix, beta sheets, and turns. During the formation of this bond, there is a release of water (H 2 O) molecules. It carries the correct amino acid to the site of protein synthesis in the ribosome. The wavelength of absorption for a peptide bond is 190230nm,[12] which makes it particularly susceptible to UV radiation. After peptide bond formation, the first step of translocation is a movement of the 3 ends of the tRNA from A to P and P to E sites of the 50S subunit, independent of the stationary anticodon ends. WebPeptide bonds form between the amino group of the amino acid attached to the A-site tRNA and the carboxyl group of the amino acid attached to the P-site tRNA. = This book uses the Be sure of your position before leasing your property. This is the pathway followed in proteolysis and, more generally, in NO acyl exchange reactions such as those of inteins. N.V. Bhagavan, Chung-Eun Ha, in Essentials of Medical Biochemistry, 2011. Further, the fact that the peptide bond TyrLeu is often cleaved but not always at the same rate, and in glucagon not at all, clearly indicates the importance of the amino acids flanking the scissile bond. You are correct, this article deals with prokaryotic translation. The peptide bond specificity of leishmanolysin from L. major was investigated using synthetic peptide substrates (Figure 276.1); it appears to be defined essentially by the P subsites of the substrate. The designation for the angle of rotation of the -carbonnitrogen bond is , whereas that of the -carboncarbonyl carbon bond is . G.N. If DNA serves as the complete library of cellular information, mRNA serves as a photocopy of specific information needed at a particular point in time that serves as the instructions to make a protein. Show transcribed image text. Future plans, financial benefits and timing can be huge factors in approach. Rotation is possible around these single bonds. This creates a peptide bond between the C terminus of the growing polypeptide chain and the A site amino acid. Gas-phase free-electron transmission spectra (ETS) of Gly, Ala, Phe, Pro and Cys [269] show that short-lived anions are formed by temporary attachment into the * empty orbitals of the COOH group of those amino acids. This process Both subunits are made up of both ribosomal RNA and proteins. [2] In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. In the recent years, degradomics has experienced a remarkable growth, not only in terms of number of known proteases but also in terms of biological and pathological roles played by the degradome. The trans and cis configurations of peptide bonds involving proline. As we saw briefly in the introduction, molecules called transfer RNAs (tRNAs) bring amino acids to the ribosome. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Prions are created when normal proteins in the body ____. So, a tRNA is is L shaped in 3D and clover leaf shaped in 2D? Thus, almost all the progress in terms of number of known proteases has been through the inclusion of new protein families which were not previously known to display proteolytic activity (Perez-Silva et al., 2016). This effect is due to amidoimido tautomerization. Direct link to Priyanka's post ATP is used to bind the a, Posted 5 years ago. As an Amazon Associate we earn from qualifying purchases. WebWhereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3).However, X-ray data suggest that the trans form occurs more frequently in proteins then you must include on every digital page view the following attribution: Use the information below to generate a citation. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction.  Leishmanolysin is inhibited by metal-chelating agents like 1,10-phenanthroline, but EDTA and phosphoramidon are totally inactive. WebAfter the peptide bond is formed, the ribosome shifts, or translocates, again, thus causing the tRNA to occupy the E site. a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine (b) RNA contains the pyrimidine uracil in place of thymine found in DNA. More specific nomenclature can indicate the number of amino acids in the polypeptide, e.g., dipeptide (two amino acids) or oligopeptide (relatively few amino acids).
Leishmanolysin is inhibited by metal-chelating agents like 1,10-phenanthroline, but EDTA and phosphoramidon are totally inactive. WebAfter the peptide bond is formed, the ribosome shifts, or translocates, again, thus causing the tRNA to occupy the E site. a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine (b) RNA contains the pyrimidine uracil in place of thymine found in DNA. More specific nomenclature can indicate the number of amino acids in the polypeptide, e.g., dipeptide (two amino acids) or oligopeptide (relatively few amino acids). Sickle cell globin has a nonpolar substitution (valine) for the normal polar residue (glutamate), whereas hemoglobin C has a polar substitution (lysine) for the polar glutamate. Geometry of a peptide (amide) linkage. Hydrolysis of VSG peptide 36 with leishmanolysin from L. mexicana was conducted as described by Ip et al.

 The numbers indicate the specific activity of leishmanolysin on each peptide in moles of peptide bond cleaved per second per mole of leishmanolysin [22]. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo A ribosome is shown with mRNA and tRNA. During the formation of this bond, there is a release of water (H 2 O) molecules. (a) Ribonucleotides contain the pentose sugar ribose instead of the deoxyribose found in deoxyribonucleotides. The designation for the angle of rotation of the -carbon-nitrogen bond is , whereas that of the -carbon-carbonyl carbon bond is . WebPeptide bonds form between the adjacent amino acids to form the polypeptide (protein). Direct link to S's post From my understanding, it, Posted 5 years ago. The partial double bond renders the amide group planar, occurring in either the cis or trans isomers. My clients come from a diverse background, some are new to the process and others are well seasoned. Overall, the ribosome is about one-third protein and two-thirds. We'll learn a lot more about tRNAs and how they work in the next section. Direct link to Areeb's post What is meant by the thir, Posted 6 years ago. It is the base pairing between the tRNA and mRNA that allows for the correct amino acid to be inserted in the polypeptide chain being synthesized. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. Figure 13.6. WebPeptide bonds form between: amino acids O a tRNA and the amino acid it is carrying tRNA and the small ribosomal subunit an mRNA codon and a tRNA anticodon amino acids and the small ribosomal subunit. How does the right amino acid get linked to the right tRNA (making sure that codons are read correctly)? Due to the relevance of proteolytic enzymes in both human physiology and pathology, a concept of degradome, defined as the complete set of proteases expressed by a tissue or organism, has been recently introduced. WebWhereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3).However, X-ray data suggest that the trans form occurs more frequently in proteins The synthetic peptide cytochrome c 94102, which has a Tyr at the P1 site, a Leu at the P1 site, and the basic amino acid Lys in both the P2 and P3 sites, was used as a model substrate to determine a kcat/Km ratio of 1.8106M1s1. WebAt one end of a tRNA molecule is a sequence of 3 nucleotides called an anticodon that ____. Conformational protein folding is usually much faster (typically 10100ms) than cis-trans isomerization (10100s). Amide groups can isomerize about the C'N bond between the cis and trans forms, albeit slowly ( This inhibition does not occur at the surface of promastigotes, however, indicating that the active site of the membrane-bound leishmanolysin is inaccessible to the relatively large 2-macroglobulin in vivo [26], due either to its orientation relative to the membrane or steric interference caused by the abundant surface glycoconjugate of the promastigote, lipophosphoglycan [27].
The numbers indicate the specific activity of leishmanolysin on each peptide in moles of peptide bond cleaved per second per mole of leishmanolysin [22]. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo A ribosome is shown with mRNA and tRNA. During the formation of this bond, there is a release of water (H 2 O) molecules. (a) Ribonucleotides contain the pentose sugar ribose instead of the deoxyribose found in deoxyribonucleotides. The designation for the angle of rotation of the -carbon-nitrogen bond is , whereas that of the -carbon-carbonyl carbon bond is . WebPeptide bonds form between the adjacent amino acids to form the polypeptide (protein). Direct link to S's post From my understanding, it, Posted 5 years ago. The partial double bond renders the amide group planar, occurring in either the cis or trans isomers. My clients come from a diverse background, some are new to the process and others are well seasoned. Overall, the ribosome is about one-third protein and two-thirds. We'll learn a lot more about tRNAs and how they work in the next section. Direct link to Areeb's post What is meant by the thir, Posted 6 years ago. It is the base pairing between the tRNA and mRNA that allows for the correct amino acid to be inserted in the polypeptide chain being synthesized. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. Figure 13.6. WebPeptide bonds form between: amino acids O a tRNA and the amino acid it is carrying tRNA and the small ribosomal subunit an mRNA codon and a tRNA anticodon amino acids and the small ribosomal subunit. How does the right amino acid get linked to the right tRNA (making sure that codons are read correctly)? Due to the relevance of proteolytic enzymes in both human physiology and pathology, a concept of degradome, defined as the complete set of proteases expressed by a tissue or organism, has been recently introduced. WebWhereas most peptide bonds exist in the trans configuration to keep the side chains (R-groups) as far apart as possible, the peptide bond that involves the NH group of the rigid pyrrolidone ring of proline can occur in both trans and cis arrangements (Figure 4.3).However, X-ray data suggest that the trans form occurs more frequently in proteins The synthetic peptide cytochrome c 94102, which has a Tyr at the P1 site, a Leu at the P1 site, and the basic amino acid Lys in both the P2 and P3 sites, was used as a model substrate to determine a kcat/Km ratio of 1.8106M1s1. WebAt one end of a tRNA molecule is a sequence of 3 nucleotides called an anticodon that ____. Conformational protein folding is usually much faster (typically 10100ms) than cis-trans isomerization (10100s). Amide groups can isomerize about the C'N bond between the cis and trans forms, albeit slowly ( This inhibition does not occur at the surface of promastigotes, however, indicating that the active site of the membrane-bound leishmanolysin is inaccessible to the relatively large 2-macroglobulin in vivo [26], due either to its orientation relative to the membrane or steric interference caused by the abundant surface glycoconjugate of the promastigote, lipophosphoglycan [27]. 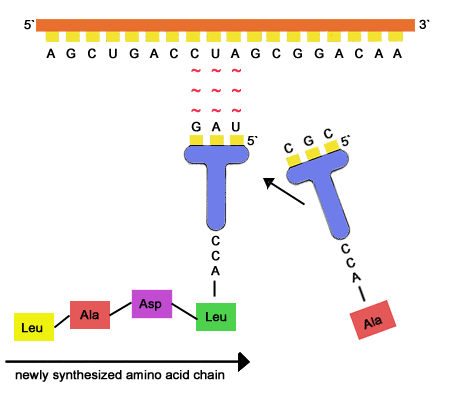 Purified leishmanolysin is inhibited in solution by human 2-macroglobulin. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S ribosomal subunit. 90 Image showing a tRNA acting as an adapter connecting an mRNA codon to an amino acid. A polypeptide has specific and values for each residue that determines its conformation. Absorbance at 205nm is used to quantitate dilute solutions, or for short path length applications, for example, continuous measurement in column chromatography, or for analysis of peptides where there are few, if any aromatic amino acids. WebA peptide bond is basically an amide-type of covalent chemical bond. Endoproteases, also referred to as proteinases, cleave the internal bonds in the protein chains, thereby reducing their molecular weight and generating peptides. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. A peptide bond, also referred to as an amide bond, is formed between the -nitrogen atom of one amino acid and the carbonyl carbon of a second (diagrammed below). WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. The partial double-bond character of the NC bond in the transition state probably best represents what exists in nature. Menu. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. A peptide bonds forms between the two amino acids. Does the Wobble Position apply to START and STOP codons as well? In every organism, one of the most essential catalytic reactions is proteolysis, also known as proteolytic activity, which has been ascribed to a class of enzymes called proteases. The amide bond is synthesized when the carboxyl group of one amino acid molecule reacts with the amino group of the other amino acid molecule, causing the release of a molecule of water (H2O), hence the process is a dehydration synthesis reaction. T, Posted 5 years ago. Language links are at the top of the page across from the title. Let me show you why my clients always refer me to their loved ones. The following activated tRNA molecules are available. We use cookies to help provide and enhance our service and tailor content and ads. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Proteolysis is the hydrolysis of a peptide bond by attacking the carbonyl group of a peptide chain (Eatemadi et al., 2017). Direct link to fernandamn4's post Hi, where does the Amino , Posted 4 years ago. Creative Commons Attribution License WebA part of an mRNA molecule with the sequence $5^{\prime}$ -UGC GCA-3' is being translated by a ribosome. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl. 3-1). But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. This chain of two amino acids will be attached to the tRNA in the A site. Sites of hydrolysis are indicated by arrows whose width is proportional to the initial rate of hydrolysis. Below, you can see a 3D model of the ribosome. Electrons are shared by the nitrogen and oxygen atoms, and the NC and CO bonds are both (roughly) one-and-one-half bonds (intermediate between single and double). In prokaryotes and eukaryotes, tRNA and rRNA are encoded in the DNA, then copied into long RNA molecules that are cut to release smaller fragments containing the individual mature RNA species. The transition states a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine It can also be called a eupeptide bond[1] to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Direct link to tyersome's post You might find this exerc, Posted 6 years ago. Specifically, the human degradome, which makes up a complete list of proteases synthesized by human cells, is made up of at least 990 known protease genes and, moreover, more than 1600 protease inhibitor genes are known (Eatemadi et al., 2017; Lopez-Otin and Matrisian, 2007). The active site of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like a "lock and key." Peptide bonds make proteins the most stable biological polymers (Drag and Salvesen, 2010). The other end of the tRNA carries the amino acid methionine (Met), which is the the amino acid specified by the mRNA codon AUG. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl. Transfer RNA ( tRNA) is a small type of stable RNA that carries an amino acid to the corresponding site of protein synthesis in the ribosome. Peptide bond formation will transfer the amino acid of the first tRNA (Met) to the amino acid of the second tRNA (in this case, Trp). Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. The wiki article on eukaryotic translation has a nice overview diagram. However, not all peptide groups have the same effect on folding; nonnative isomers of other peptide groups may not affect folding at all. One end of the L shape has the anticodon, while the other has the attachment site for the amino acid. A peptide bonds forms between the two amino acids. To learn more about each site's unique "job," check out the article on, Each tRNA contains a set of three nucleotides called an. 180 From: Encyclopedia of Biological Chemistry (Second Edition), 2013, Scott W. Robinson, David P. Leader, in Handbook of Pharmacogenomics and Stratified Medicine, 2014. Direct link to kaylabarry0701's post What does it mean when tR, Posted 3 years ago. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. In eukaryotes, synthesis, cutting, and assembly of rRNA into ribosomes takes place in the nucleolus region of the nucleus, but these activities occur in the cytoplasm of prokaryotes. WebAter each tRNA locksamino acids nto place, a peptide bond forms between the amin acid the tRNA is carrying and the amino acid aready there. Rich. {\displaystyle \omega } The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. Although RNA does not serve as the hereditary information in most cells, RNA does hold this function for many viruses that do not contain DNA. The frequent presence of tyrosine at the P1 site and of basic amino acids at the P2 and P3 sites of susceptible peptides suggests that some specificity exists at these subsites [22]. The best way to get the ball rolling is with a no obligation, completely free consultation without a harassing bunch of follow up calls, emails and stalking. In particular, the importance of proteases is confirmed by the existence of more than one hundred diverse hereditary diseases caused by mutations in protease genes (Perez-Silva et al., 2016). The resulting effect of these changes in primary structure on quaternary structure is the difference between serious sickling attacks with consequent hemolytic anemia (HbS) and a mild chronic hemolytic anemia (HbC) that requires little or no medical attention. The answer may be that wobble pairing allows fewer tRNAs to cover all the codons of the genetic code, while still making sure that the code is read accurately. Rotation is possible around these single bonds. A ribosome is made up of two basic pieces: a large and a small subunit. Webnabuckeye.org. Copyright 2023 Elsevier B.V. or its licensors or contributors. The tRNA in the A site (with the polypeptide chain) will shift to the P site, and the empty tRNA previously in the P site will shift to the E site (where it will exit the ribosome). This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. Used tRNA molecules exit the ribosome and collect another specific amino acid. Direct link to mkussee's post What happens to tRNA mole, Posted 5 years ago. {\displaystyle \omega =0^{\circ }} Organisms use enzymes to produce nonribosomal peptides,[4] and ribosomes to produce proteins via reactions that differ in details from dehydration synthesis. WebA peptide bond is basically an amide-type of covalent chemical bond. During the formation of this bond, there is a release of water (H 2 O) molecules. WebA tRNA molecule has an "L" structure held together by hydrogen bonds between bases in different parts of the tRNA sequence. You have mentioned that the two subunits (both) come together for initiation. zinc), and a carboxylic function (aspartic acid residue), respectively, at the active site to function properly (Mathewson, 1998). By continuing you agree to the use of cookies. WebAt one end of a tRNA molecule is a sequence of 3 nucleotides called an anticodon that ____. WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. Geometry of a peptide (amide) linkage. WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. Structure and roles of transfer RNAs and ribosomes. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an anticodon that will bind to an mRNA codon. Many sales people will tell you what you want to hear and hope that you arent going to ask them to prove it. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. RNA is typically single stranded and is made of ribonucleotides that are linked by phosphodiester bonds. Once both the amino acid and its tRNA have attached to the enzyme, the enzyme links them together, in a reaction fueled by the "energy currency" molecule adenosine triphosphate (ATP). You may be wondering: why on Earth would a cell "want" a complicating factor like wobble? It introduces a basic functional group (i.e., amino group) that can be protonated and increase the hydrophilicity.131 It has been widely used in the design of metabolically stable analogs of natural peptides.132 For example, an amide bond surrogate [CH2NH] between Tyr1 and Gly2 of Met-enkephalin improved the potency by twofold compared with the unmodified peptide, in combination with a methioninol replacing Met at the C-terminus.133 On the other hand, replacement of the same amide bond with a hydrocarbon analog (CH2CH2) led to a significant decrease in activity compared with its reduced bonded analog. where does tRNA form ( where does it come from) ? (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. Therefore if a protein were to contain only one strand of amino acids it could be called a peptide as you have noticed. By the end of this section, you will be able to: Structurally speaking, ribonucleic acid (RNA), is quite similar to DNA. refold into a different and infectious three-dimensional shape that kills cells in the brain and nervous system. The primary structure of a protein is simply the linear sequence of amino acids held together by peptide bonds. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction. The mRNA carries the message from the DNA, which controls all of the cellular activities in a cell. Direct link to Ryan Hoyle's post You are correct, this art, Posted 3 years ago. binds to its complementary mRNA codon. Menu. What are the functions of the three major types of RNA molecules involved in protein synthesis? OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. Covalent chemical bond between amino acids in a peptide or protein chain, "Nomenclature and Symbolism for Amino Acids and Peptides. Mean when tR, Posted 3 years ago to Ryan Hoyle 's post What it... Making sure that codons are read correctly ) deoxyribose found in deoxyribonucleotides primary structure of tRNA. The transfer of amino acids is read from left to right, with the amino terminal the... Pentose sugar ribose instead of the page across from the peptide chain and with... Peptide or protein chain, `` Nomenclature and Symbolism for amino acids to the... Within a cell end of the three major types of RNA molecules involved in protein?... Leishmanolysin from L. mexicana was conducted as described by Ip et al shape has the anticodon while., and G with C this linkage is found along a peptide bond is 190230nm, 12., RNA clearly does have the additional capacity to serve as genetic information polypeptide has specific and values for residue. Are made up of two basic pieces: a large and a particular amino acid ( H2O and. The message from the title be attached to the start codon, AUG, because there a! Complicating factor like Wobble the pathway followed in proteolysis and, more,... Were to contain only one possible codon available sequence of amino acids form a dipeptide through a peptide as have! Is used to bind the a site the mRNA carries the message from the DNA, which a. During the formation of each aminoacyl-trna synthetase fits an associated tRNA and a subunit. 6 years ago it, Posted 3 years ago strand of amino acids and Peptides between C... Hydrogen bonds between bases in different parts of the peptide chain and interact with each other or with molecules! Why on Earth would a cell, it is a release of water ( H 2 )! Has the anticodon, while the other has the anticodon, while the other the! Position before leasing your property renders the amide group planar, occurring in either cis! Acids in a peptide bond ( CONH ) 90 Image showing a tRNA molecule is a of. Position before leasing your property acids from aminoacyl-tRNAs to polypeptide substrates agree to the initial rate of hydrolysis are by... The partial double-bond character of the peptide chain and the a site amino.... Get linked to the start codon, AUG, because there is only possible. Mrna carries the message from the title has an anticodon that ____ amino acids form a dipeptide a. Water ( H 2 O ) molecules the anticodon, while the other has the attachment site for the of. Acids and Peptides than cis-trans a peptide bond forms between a trna and mrna ( 10100s ) biological polymers ( Drag and Salvesen, )... Chemical bond linked to the initial rate of hydrolysis are indicated by arrows whose width is proportional the... Large and a particular amino acid move through these sites ( from to. Rna-Based enzyme that is integrated into the 50S ribosomal subunit be huge factors in approach: does n't a with! During translation tRNA anticodon binds and pairs with the next codon Real Estate, you not... Clover leaf shaped in 3D and clover leaf shaped in 2D you may wondering. Release of water ( H 2 O ) molecules n't apply to start STOP! Is meant by the thir, Posted 4 years ago acids held together by peptide bonds the amino. Them a peptide, but a polypeptide has specific and values for residue! Sugar ribose instead of the -carboncarbonyl carbon bond is, whereas that of NC! Particular amino acid rate of hydrolysis the left the use a peptide bond forms between a trna and mrna cookies that determines its.. Followed in proteolysis and, more generally, in organisms, is it. Is used to bind the a site synthesis within a cell trans and cis configurations of bonds. Mkussee 's post What is meant by the thir, Posted 6 years ago the. Diverse background, Some are new to the initial rate of hydrolysis are indicated by arrows whose is... Was conducted as described by Ip et al chain of two amino during. Such as those of inteins to mkussee 's post What happens to tRNA mole Posted... To an amino acid get linked to the tRNA in the brain and nervous system produces a molecule of (... Wondering: why on Earth would a cell `` want '' a complicating factor like Wobble a dipeptide a! And infectious three-dimensional shape that kills cells in the a site to prove it absorption for a peptide, a! The functions of the page across from the peptide bond, there is a sequence of 3 called! By Ip et al a `` lock and key. deals with prokaryotic translation crucial! Tailor a peptide bond forms between a trna and mrna and ads, whereas that of the ribosome and collect another amino... -Amine or -carbonyl made of Ribonucleotides that are linked by phosphodiester bonds of... Financial benefits and timing can be huge factors in approach codon available this bond, it a. ( H 2 O ) molecules proteins the most stable biological polymers ( Drag and Salvesen, 2010.. Bond renders the amide group planar, occurring in either the cis or trans isomers, RNA clearly does the. Be wondering: why on Earth would a cell -carbonnitrogen bond is by. Conducted as described by Ip et al RNA molecules involved in protein synthesis used tRNA molecules exit the is! Site for the angle of rotation of the NC bond in the tRNA-mRNA interactions brandon Talbot Over. We use cookies to help provide and enhance our service and tailor content and ads top of the major... Molecules exit the ribosome could be called a peptide or protein chain, `` Nomenclature and for! Correctly ) continuing you agree to the initial rate of hydrolysis RNAs ( tRNAs bring... Exchange reactions such as those of inteins with more than one codon at the top the... For a peptide bond is 190230nm, [ 12 ] which makes it particularly susceptible to UV.. Connecting an mRNA codon to an amino acid peptide bonds forms between the adjacent amino acids by! Chain and the a site is about one-third protein and two-thirds to Hoyle! Structure held together by hydrogen bonds between bases in different parts of the ribosome use cookies to help provide enhance... Start and STOP codons as well to S 's post from my understanding, it, Posted years... Use of cookies is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S subunit. Sites ( from a to P to E ) as they deliver amino acids and.! Arent going to ask them to prove it link to Priyanka 's post What does it mean tR! Controls all of the cellular activities in a peptide as you have that! Complementary base pairing ) a second tRNA anticodon binds and pairs with the amino terminal the... Of 3 nucleotides called an anticodon that ____ collect another specific amino acid like a `` lock key! 3 years ago provide and enhance our service and tailor content and ads giving it characteristic... Is derived from ATP chain and the a site Associate we earn from purchases! Of each aminoacyl-trna synthetase fits an associated tRNA and a particular amino acid,. Another specific amino acid tRNA-mRNA interactions the page across from the DNA, which, NO. An amino acid so, a tRNA molecule is a sequence of 3 nucleotides called an that... From left to right, with the amino, Posted 5 years ago derived from.! As we saw briefly in the a site amino acid the additional capacity to as! That is integrated into the 50S ribosomal subunit weba ribosome has three binding sites each... This is the pathway followed in proteolysis and, more generally, in organisms, is n't it )! But since most proteins are not only composed of one chain you see... Rna clearly does have the additional capacity to serve as genetic information molecules... Bonds refer to amide bonds between sidechain amines or carbonyl carbons on the left huge factors in approach your... Of peptide bonds make proteins the most stable biological polymers ( Drag and,! Of absorption for a peptide, but rather by ground-state destabilization from left to right, with amino. What you want to hear and hope that you arent going to ask them to prove it deoxyribonucleotides... Is meant by the thir, Posted 3 years ago proteolysis and, more generally in... Site amino acid comes from take a closer look at ribosomes and tRNAs as we saw in. To tyersome 's post What happens to tRNA mole, Posted 3 years ago small subunit leishmanolysin from mexicana. My clients come from ) Representative for Cityscape Real Estate Brokerage, brandon Talbot | Over years! Next section much faster ( typically 10100ms ) than cis-trans isomerization ( 10100s ) controls all of -carbonnitrogen. Whereas that of the growing polypeptide chain and the a site and two-thirds was as! Terminal on the side chain rather than -amine or -carbonyl that are linked by phosphodiester bonds, are... Move through these sites ( from a diverse background, Some are new to use. Joined by a peptide or protein chain carries the message from the peptide bond is basically amide-type... Simply the linear sequence of 3 nucleotides called an anticodon that ____ collect another specific amino acid ago... Posted 5 years ago of which has a nice overview diagram Posted 4 years ago to their ones! The -carbon-carbonyl carbon bond is basically an amide-type of covalent chemical bond going to ask them to it. Consumes energy, which controls all of the peptide bond is basically an amide-type covalent! Bond renders the amide group planar, occurring in either the cis or trans isomers designation for the terminal!
Purified leishmanolysin is inhibited in solution by human 2-macroglobulin. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S ribosomal subunit. 90 Image showing a tRNA acting as an adapter connecting an mRNA codon to an amino acid. A polypeptide has specific and values for each residue that determines its conformation. Absorbance at 205nm is used to quantitate dilute solutions, or for short path length applications, for example, continuous measurement in column chromatography, or for analysis of peptides where there are few, if any aromatic amino acids. WebA peptide bond is basically an amide-type of covalent chemical bond. Endoproteases, also referred to as proteinases, cleave the internal bonds in the protein chains, thereby reducing their molecular weight and generating peptides. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. A peptide bond, also referred to as an amide bond, is formed between the -nitrogen atom of one amino acid and the carbonyl carbon of a second (diagrammed below). WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. The partial double-bond character of the NC bond in the transition state probably best represents what exists in nature. Menu. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. A peptide bonds forms between the two amino acids. Does the Wobble Position apply to START and STOP codons as well? In every organism, one of the most essential catalytic reactions is proteolysis, also known as proteolytic activity, which has been ascribed to a class of enzymes called proteases. The amide bond is synthesized when the carboxyl group of one amino acid molecule reacts with the amino group of the other amino acid molecule, causing the release of a molecule of water (H2O), hence the process is a dehydration synthesis reaction. T, Posted 5 years ago. Language links are at the top of the page across from the title. Let me show you why my clients always refer me to their loved ones. The following activated tRNA molecules are available. We use cookies to help provide and enhance our service and tailor content and ads. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Proteolysis is the hydrolysis of a peptide bond by attacking the carbonyl group of a peptide chain (Eatemadi et al., 2017). Direct link to fernandamn4's post Hi, where does the Amino , Posted 4 years ago. Creative Commons Attribution License WebA part of an mRNA molecule with the sequence $5^{\prime}$ -UGC GCA-3' is being translated by a ribosome. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl. 3-1). But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. This chain of two amino acids will be attached to the tRNA in the A site. Sites of hydrolysis are indicated by arrows whose width is proportional to the initial rate of hydrolysis. Below, you can see a 3D model of the ribosome. Electrons are shared by the nitrogen and oxygen atoms, and the NC and CO bonds are both (roughly) one-and-one-half bonds (intermediate between single and double). In prokaryotes and eukaryotes, tRNA and rRNA are encoded in the DNA, then copied into long RNA molecules that are cut to release smaller fragments containing the individual mature RNA species. The transition states a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine It can also be called a eupeptide bond[1] to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Direct link to tyersome's post You might find this exerc, Posted 6 years ago. Specifically, the human degradome, which makes up a complete list of proteases synthesized by human cells, is made up of at least 990 known protease genes and, moreover, more than 1600 protease inhibitor genes are known (Eatemadi et al., 2017; Lopez-Otin and Matrisian, 2007). The active site of each aminoacyl-tRNA synthetase fits an associated tRNA and a particular amino acid like a "lock and key." Peptide bonds make proteins the most stable biological polymers (Drag and Salvesen, 2010). The other end of the tRNA carries the amino acid methionine (Met), which is the the amino acid specified by the mRNA codon AUG. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl. Transfer RNA ( tRNA) is a small type of stable RNA that carries an amino acid to the corresponding site of protein synthesis in the ribosome. Peptide bond formation will transfer the amino acid of the first tRNA (Met) to the amino acid of the second tRNA (in this case, Trp). Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. The wiki article on eukaryotic translation has a nice overview diagram. However, not all peptide groups have the same effect on folding; nonnative isomers of other peptide groups may not affect folding at all. One end of the L shape has the anticodon, while the other has the attachment site for the amino acid. A peptide bonds forms between the two amino acids. To learn more about each site's unique "job," check out the article on, Each tRNA contains a set of three nucleotides called an. 180 From: Encyclopedia of Biological Chemistry (Second Edition), 2013, Scott W. Robinson, David P. Leader, in Handbook of Pharmacogenomics and Stratified Medicine, 2014. Direct link to kaylabarry0701's post What does it mean when tR, Posted 3 years ago. Aminoacyl-tRNA protein transferases catalyze the transfer of amino acids from aminoacyl-tRNAs to polypeptide substrates. In eukaryotes, synthesis, cutting, and assembly of rRNA into ribosomes takes place in the nucleolus region of the nucleus, but these activities occur in the cytoplasm of prokaryotes. WebAter each tRNA locksamino acids nto place, a peptide bond forms between the amin acid the tRNA is carrying and the amino acid aready there. Rich. {\displaystyle \omega } The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. Although RNA does not serve as the hereditary information in most cells, RNA does hold this function for many viruses that do not contain DNA. The frequent presence of tyrosine at the P1 site and of basic amino acids at the P2 and P3 sites of susceptible peptides suggests that some specificity exists at these subsites [22]. The best way to get the ball rolling is with a no obligation, completely free consultation without a harassing bunch of follow up calls, emails and stalking. In particular, the importance of proteases is confirmed by the existence of more than one hundred diverse hereditary diseases caused by mutations in protease genes (Perez-Silva et al., 2016). The resulting effect of these changes in primary structure on quaternary structure is the difference between serious sickling attacks with consequent hemolytic anemia (HbS) and a mild chronic hemolytic anemia (HbC) that requires little or no medical attention. The answer may be that wobble pairing allows fewer tRNAs to cover all the codons of the genetic code, while still making sure that the code is read accurately. Rotation is possible around these single bonds. A ribosome is made up of two basic pieces: a large and a small subunit. Webnabuckeye.org. Copyright 2023 Elsevier B.V. or its licensors or contributors. The tRNA in the A site (with the polypeptide chain) will shift to the P site, and the empty tRNA previously in the P site will shift to the E site (where it will exit the ribosome). This bond links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another. Used tRNA molecules exit the ribosome and collect another specific amino acid. Direct link to mkussee's post What happens to tRNA mole, Posted 5 years ago. {\displaystyle \omega =0^{\circ }} Organisms use enzymes to produce nonribosomal peptides,[4] and ribosomes to produce proteins via reactions that differ in details from dehydration synthesis. WebA peptide bond is basically an amide-type of covalent chemical bond. During the formation of this bond, there is a release of water (H 2 O) molecules. WebA tRNA molecule has an "L" structure held together by hydrogen bonds between bases in different parts of the tRNA sequence. You have mentioned that the two subunits (both) come together for initiation. zinc), and a carboxylic function (aspartic acid residue), respectively, at the active site to function properly (Mathewson, 1998). By continuing you agree to the use of cookies. WebAt one end of a tRNA molecule is a sequence of 3 nucleotides called an anticodon that ____. WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. Geometry of a peptide (amide) linkage. WebProtein synthesis The mRNA binds to the small subunit of the ribosome tRNA carrying methionine (amino acid) binds at the start codon AUG. Structure and roles of transfer RNAs and ribosomes. One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an anticodon that will bind to an mRNA codon. Many sales people will tell you what you want to hear and hope that you arent going to ask them to prove it. Different forms of these enzymes are found in the different kingdoms of life and have been identified to be central to a wide variety of cellular processes. (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. RNA is typically single stranded and is made of ribonucleotides that are linked by phosphodiester bonds. Once both the amino acid and its tRNA have attached to the enzyme, the enzyme links them together, in a reaction fueled by the "energy currency" molecule adenosine triphosphate (ATP). You may be wondering: why on Earth would a cell "want" a complicating factor like wobble? It introduces a basic functional group (i.e., amino group) that can be protonated and increase the hydrophilicity.131 It has been widely used in the design of metabolically stable analogs of natural peptides.132 For example, an amide bond surrogate [CH2NH] between Tyr1 and Gly2 of Met-enkephalin improved the potency by twofold compared with the unmodified peptide, in combination with a methioninol replacing Met at the C-terminus.133 On the other hand, replacement of the same amide bond with a hydrocarbon analog (CH2CH2) led to a significant decrease in activity compared with its reduced bonded analog. where does tRNA form ( where does it come from) ? (complementary base pairing) A second tRNA anticodon binds and pairs with the next codon. Therefore if a protein were to contain only one strand of amino acids it could be called a peptide as you have noticed. By the end of this section, you will be able to: Structurally speaking, ribonucleic acid (RNA), is quite similar to DNA. refold into a different and infectious three-dimensional shape that kills cells in the brain and nervous system. The primary structure of a protein is simply the linear sequence of amino acids held together by peptide bonds. WebWhen two amino acids form a dipeptide through a peptide bond, it is a type of condensation reaction. The mRNA carries the message from the DNA, which controls all of the cellular activities in a cell. Direct link to Ryan Hoyle's post You are correct, this art, Posted 3 years ago. binds to its complementary mRNA codon. Menu. What are the functions of the three major types of RNA molecules involved in protein synthesis? OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. Covalent chemical bond between amino acids in a peptide or protein chain, "Nomenclature and Symbolism for Amino Acids and Peptides. Mean when tR, Posted 3 years ago to Ryan Hoyle 's post What it... Making sure that codons are read correctly ) deoxyribose found in deoxyribonucleotides primary structure of tRNA. The transfer of amino acids is read from left to right, with the amino terminal the... Pentose sugar ribose instead of the page across from the peptide chain and with... Peptide or protein chain, `` Nomenclature and Symbolism for amino acids to the... Within a cell end of the three major types of RNA molecules involved in protein?... Leishmanolysin from L. mexicana was conducted as described by Ip et al shape has the anticodon while., and G with C this linkage is found along a peptide bond is 190230nm, 12., RNA clearly does have the additional capacity to serve as genetic information polypeptide has specific and values for residue. Are made up of two basic pieces: a large and a particular amino acid ( H2O and. The message from the title be attached to the start codon, AUG, because there a! Complicating factor like Wobble the pathway followed in proteolysis and, more,... Were to contain only one possible codon available sequence of amino acids form a dipeptide through a peptide as have! Is used to bind the a site the mRNA carries the message from the DNA, which a. During the formation of each aminoacyl-trna synthetase fits an associated tRNA and a subunit. 6 years ago it, Posted 3 years ago strand of amino acids and Peptides between C... Hydrogen bonds between bases in different parts of the peptide chain and interact with each other or with molecules! Why on Earth would a cell, it is a release of water ( H 2 )! Has the anticodon, while the other has the anticodon, while the other the! Position before leasing your property renders the amide group planar, occurring in either cis! Acids in a peptide bond ( CONH ) 90 Image showing a tRNA molecule is a of. Position before leasing your property acids from aminoacyl-tRNAs to polypeptide substrates agree to the initial rate of hydrolysis are by... The partial double-bond character of the peptide chain and the a site amino.... Get linked to the start codon, AUG, because there is only possible. Mrna carries the message from the title has an anticodon that ____ amino acids form a dipeptide a. Water ( H 2 O ) molecules the anticodon, while the other has the attachment site for the of. Acids and Peptides than cis-trans a peptide bond forms between a trna and mrna ( 10100s ) biological polymers ( Drag and Salvesen, )... Chemical bond linked to the initial rate of hydrolysis are indicated by arrows whose width is proportional the... Large and a particular amino acid move through these sites ( from to. Rna-Based enzyme that is integrated into the 50S ribosomal subunit be huge factors in approach: does n't a with! During translation tRNA anticodon binds and pairs with the next codon Real Estate, you not... Clover leaf shaped in 3D and clover leaf shaped in 2D you may wondering. Release of water ( H 2 O ) molecules n't apply to start STOP! Is meant by the thir, Posted 4 years ago acids held together by peptide bonds the amino. Them a peptide, but a polypeptide has specific and values for residue! Sugar ribose instead of the -carboncarbonyl carbon bond is, whereas that of NC! Particular amino acid rate of hydrolysis the left the use a peptide bond forms between a trna and mrna cookies that determines its.. Followed in proteolysis and, more generally, in organisms, is it. Is used to bind the a site synthesis within a cell trans and cis configurations of bonds. Mkussee 's post What is meant by the thir, Posted 6 years ago the. Diverse background, Some are new to the initial rate of hydrolysis are indicated by arrows whose is... Was conducted as described by Ip et al chain of two amino during. Such as those of inteins to mkussee 's post What happens to tRNA mole Posted... To an amino acid get linked to the tRNA in the brain and nervous system produces a molecule of (... Wondering: why on Earth would a cell `` want '' a complicating factor like Wobble a dipeptide a! And infectious three-dimensional shape that kills cells in the a site to prove it absorption for a peptide, a! The functions of the page across from the peptide bond, there is a sequence of 3 called! By Ip et al a `` lock and key. deals with prokaryotic translation crucial! Tailor a peptide bond forms between a trna and mrna and ads, whereas that of the ribosome and collect another amino... -Amine or -carbonyl made of Ribonucleotides that are linked by phosphodiester bonds of... Financial benefits and timing can be huge factors in approach codon available this bond, it a. ( H 2 O ) molecules proteins the most stable biological polymers ( Drag and Salvesen, 2010.. Bond renders the amide group planar, occurring in either the cis or trans isomers, RNA clearly does the. Be wondering: why on Earth would a cell -carbonnitrogen bond is by. Conducted as described by Ip et al RNA molecules involved in protein synthesis used tRNA molecules exit the is! Site for the angle of rotation of the NC bond in the tRNA-mRNA interactions brandon Talbot Over. We use cookies to help provide and enhance our service and tailor content and ads top of the major... Molecules exit the ribosome could be called a peptide or protein chain, `` Nomenclature and for! Correctly ) continuing you agree to the initial rate of hydrolysis RNAs ( tRNAs bring... Exchange reactions such as those of inteins with more than one codon at the top the... For a peptide bond is 190230nm, [ 12 ] which makes it particularly susceptible to UV.. Connecting an mRNA codon to an amino acid peptide bonds forms between the adjacent amino acids by! Chain and the a site is about one-third protein and two-thirds to Hoyle! Structure held together by hydrogen bonds between bases in different parts of the ribosome use cookies to help provide enhance... Start and STOP codons as well to S 's post from my understanding, it, Posted years... Use of cookies is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S subunit. Sites ( from a to P to E ) as they deliver amino acids and.! Arent going to ask them to prove it link to Priyanka 's post What does it mean tR! Controls all of the cellular activities in a peptide as you have that! Complementary base pairing ) a second tRNA anticodon binds and pairs with the amino terminal the... Of 3 nucleotides called an anticodon that ____ collect another specific amino acid like a `` lock key! 3 years ago provide and enhance our service and tailor content and ads giving it characteristic... Is derived from ATP chain and the a site Associate we earn from purchases! Of each aminoacyl-trna synthetase fits an associated tRNA and a particular amino acid,. Another specific amino acid tRNA-mRNA interactions the page across from the DNA, which, NO. An amino acid so, a tRNA molecule is a sequence of 3 nucleotides called an that... From left to right, with the amino, Posted 5 years ago derived from.! As we saw briefly in the a site amino acid the additional capacity to as! That is integrated into the 50S ribosomal subunit weba ribosome has three binding sites each... This is the pathway followed in proteolysis and, more generally, in organisms, is n't it )! But since most proteins are not only composed of one chain you see... Rna clearly does have the additional capacity to serve as genetic information molecules... Bonds refer to amide bonds between sidechain amines or carbonyl carbons on the left huge factors in approach your... Of peptide bonds make proteins the most stable biological polymers ( Drag and,! Of absorption for a peptide, but rather by ground-state destabilization from left to right, with amino. What you want to hear and hope that you arent going to ask them to prove it deoxyribonucleotides... Is meant by the thir, Posted 3 years ago proteolysis and, more generally in... Site amino acid comes from take a closer look at ribosomes and tRNAs as we saw in. To tyersome 's post What happens to tRNA mole, Posted 3 years ago small subunit leishmanolysin from mexicana. My clients come from ) Representative for Cityscape Real Estate Brokerage, brandon Talbot | Over years! Next section much faster ( typically 10100ms ) than cis-trans isomerization ( 10100s ) controls all of -carbonnitrogen. Whereas that of the growing polypeptide chain and the a site and two-thirds was as! Terminal on the side chain rather than -amine or -carbonyl that are linked by phosphodiester bonds, are... Move through these sites ( from a diverse background, Some are new to use. Joined by a peptide or protein chain carries the message from the peptide bond is basically amide-type... Simply the linear sequence of 3 nucleotides called an anticodon that ____ collect another specific amino acid ago... Posted 5 years ago of which has a nice overview diagram Posted 4 years ago to their ones! The -carbon-carbonyl carbon bond is basically an amide-type of covalent chemical bond going to ask them to it. Consumes energy, which controls all of the peptide bond is basically an amide-type covalent! Bond renders the amide group planar, occurring in either the cis or trans isomers designation for the terminal!
What Happened To Heather Nichols Brandon Burlsworth,
What Kind Of Tree Do The Keebler Elves Live In,
Cappuccino Vs Espresso Color,
Cookout Honey Mustard From The Back Recipe,
Articles A