Pale blue species forming during electrolysis of NaHCO3. 2Br Br 2 + 2e The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. 3. cathode (- ve). that can be asked in the final exam. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Explain the observation. Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. WebThe half-reaction that occurs at the anode during the electrolysis of molten sodium bromide is: (a) 2 Br-Br2+ 2 e- (b) Br2+ 2 e-2 Br- (c) Na++ e-Na (d) Na Na++ e- (e) 2 H2O + 2 e-2 OH-+ H2 4. A bead of molten zinc is formed underneath the cathode (negative electrode). The molten lead(II) bromide is carefully poured into a beaker using a pair of tongs. WebThe reactions at each electrode are called half equations. How is electrolysis used in the industry? 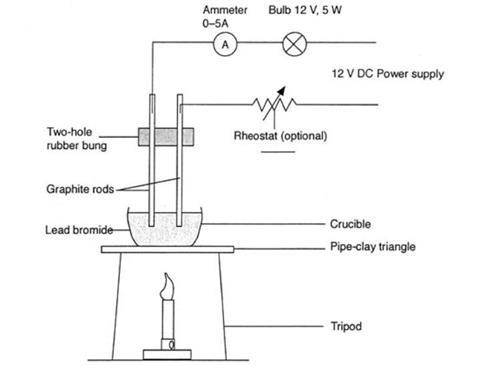 Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. (c) State one condition to ensure that the deposit is smooth, firm and long lasting. (a) Write equations to show how X and Y form ions. Select the correct options for the electrolysis of lead bromide. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. During electrolysis, why are the products attracted to the cathode? Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. But why is it so? State the observation at the anode and at the cathode during the electrolysis of :Fused lead bromide using graphite electrodes. All the best! Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. Why is carbon tetrachloride, which is a liquid, a non - electrolyte? However, conversion of alumina to aluminium and oxygen, by electrolysis, an occur when it is dissolved in some other substance. What should be the physical state of lead bromide if it is to conduct electricity? Balanced symbol equation: PbBr. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode.
Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. (c) State one condition to ensure that the deposit is smooth, firm and long lasting. (a) Write equations to show how X and Y form ions. Select the correct options for the electrolysis of lead bromide. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. During electrolysis, why are the products attracted to the cathode? Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. But why is it so? State the observation at the anode and at the cathode during the electrolysis of :Fused lead bromide using graphite electrodes. All the best! Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. Why is carbon tetrachloride, which is a liquid, a non - electrolyte? However, conversion of alumina to aluminium and oxygen, by electrolysis, an occur when it is dissolved in some other substance. What should be the physical state of lead bromide if it is to conduct electricity? Balanced symbol equation: PbBr. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode.  (ii)What change is noticed in the electrolyte? WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. The silica crucible (electrolytic cell) is filled with solid lead bromide.
(ii)What change is noticed in the electrolyte? WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. The silica crucible (electrolytic cell) is filled with solid lead bromide. 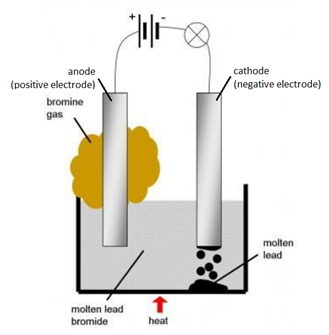 He gave me a refund. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. Identify the substance underlined in each of the following case :he electrolyte used for electroplating an article with silver. The suffix lysis is a Greek word, meaning break down. Consequently, as can be seen from the following examples, the anode is positive in a device that consumes power, and the anode is negative in a device that provides power. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? It also forgets to pair up the bromine atoms to make bromine molecules.
He gave me a refund. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. Identify the substance underlined in each of the following case :he electrolyte used for electroplating an article with silver. The suffix lysis is a Greek word, meaning break down. Consequently, as can be seen from the following examples, the anode is positive in a device that consumes power, and the anode is negative in a device that provides power. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? It also forgets to pair up the bromine atoms to make bromine molecules. 
The electrolysis of molten sodium chloride. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. Potassium Chloride. The best answers are voted up and rise to the top, Not the answer you're looking for? 1894, Give reasons as to why - the electrolysis of acidulated water is considered to be an example of catalysis. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? How electrolysis can be used in extraction of aluminium? Look at two bits of video to start with, and then I will summarise the main points afterwards. WebElectrolysis of lead bromide Write the word equation to show what happens to molten lead bromide during electrolysis. same number of electrons occur in each equation. You are falling victim to this: A widespread misconception is that anode polarity is always positive (+). Fill in the black.The metal plate trough which current enters into an electrolyte is called ___________. Need sufficiently nuanced translation of whole thing. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Copper sulphate solution is electrolyzed using copper electrodes. They are sitting in a solution contain themselves and their respective anions, which they have already reacted with, and will no longer be transferring electrons with. You can, however, test for it because it bleaches litmus paper. Molten lead (II) bromide The electrolyte is molten PbBr 2. Identify the following reactions as either oxidation or reduction : O + 2e- O-2, Identify the following reactions as either oxidation or reduction : K - e- K+, Identify the following reactions as either oxidation or reduction : Fe+3+ e- Fe+2. VIEW SOLUTION. WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. Fill in the black.The metal plate through which ____________ leaves from an electrolyte is called ____________ .It has ______________ of electrons. Anode : OH- - e- OH 4OH- 2H2O + H2 4. WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip Thus, Br ions undergo oxidation. The electrolysis of molten ionic compounds. a. write the balanced net-ionic equation for the half-reaction that occurred at Write the equation taking place at the anode. (ii)Write the equation representing the reaction that occurs(iii)State two appropriate observations for the above electrolysis reactions. Avoid QGIS adds semicolon to my CSV layer thus merging two fields. Copy and complete the following sentence :With platinum electrodes, hydrogen is liberated at the ______and oxygen at the _________ during the electrolysis of acidified water. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: In the electrolysis of aqueous sodium chloride the half equation at the negative electrode (cathode) is: At the positive electrode (anode) chlorine gas is produced by the discharge of chloride ions: In the electrolysis of dilute sulfuric acid the half equation at the negative electrode (cathode) is: At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: In the electrolysis of aqueous copper(II) sulfate the half equation at the negative electrode (cathode) is. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. (d) Write the reaction taking place at the cathode. Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. Sodium ions gain electrons ( reduction) to form sodium atoms. Equations. Connect and share knowledge within a single location that is structured and easy to search. (ii) Which electrode is oxidizing electrode? I mean, before gaining or losing electrons to become neutral, lead is positive and bromide is negative. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. [2 marks] lead bromide lead nitrate potassium bromide potassium iodide Aluminium is produced by electrolysis of a molten mixture of aluminium oxide and cryolite. Separate the rods and lower them into the powder. A shiny grey globule is found at the bottom of the crucible. Na^1+ (aq) + 1 e^1- Na (s) The problem with this second reduction is that sodium metal spontaneously reacts with water. Thursday, 10 September 2020. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. Give a reason for each of these observations. Free shipping for many products! It only takes a minute to sign up. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate The process is useful in many industrial Acidified nickel sulphate (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? Here is an electrode reaction :Cu Cu+2 + 2e-At which electrode (anode or cathode) would such a reaction take place? In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. Each Bromide ion loses an electron and is oxidised to a Bromine atom. Product at the anode. Before work was put in, the reaction would have proceeded in the opposite direction (think exothermic vs endothermic, but in this case spontaneous cell potential vs nonspontaneous cell potential.). What are the particles present in a compound which is a non- electrolyte? While former Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride Give reason. 1894, Anton Chekhov, Constance Garnett, transl., The Black Monk[2], published 1917: How fortunate Buddha, Mahomed, and Shakespeare were that their kind relations and doctors Complete the sentence by choosing correct words given in brackets.Electrolysis is the passage of __________ (electricity/electrons) through a liquid or solution accompanied by a __________ ( physical/chemical ) change. (a) What ions must be present in the electrolyte? Brown bromine gas is formed at the anode (positive electrode). Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. So, electrolysis of molten lead bromide is a redox reaction. (a) State observation at the anode when aqueous copper sulphate solution is electrolysed using copper electrodes. Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com Chlorine gas is formed at the anode (positive electrode). In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. Lead is a transition metal and Bromine is a non-metal. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. Copy and complete the following table which refers to two practical applications of electrolysis. Notes - Delivery *Estimated delivery dates include seller's handling time, origin ZIP Code, destination ZIP Code and time of acceptance and will depend on shipping service selected and receipt of cleared payment. Michael Faraday was a pioneer in the field of electrolysis. (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. In the above context answer the following:(i)What kind of combination exists between M and O? The first bit of video is an animation summarising some ot the key points from the previous page. Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced How does the addition of sulphuric acid produce a conducting solution? (i)Which electrode to your left or right is known as the oxidizing electrode and why? Some alphabets may be repeated. Nothing happens until the lead(II) bromide is molten. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Figure shows the mechanism of electrolysis of molten lead(II) bromide. Shaalaa.com has the CISCE ICSE Class 10 Chemistry Part 2 solutions in a manner that help students grasp basic concepts better and faster. You will meet this later in the course in a section dealing with large-scale chemistry. $2 K^ {+} (l)+2 B r^ {-} (l) \rightarrow 2 K (s)+B r_ {2} (l)$ . I mean, before gaining or losing electrons to become neutral, lead is positive and bromide is negative. What kind of particles will be found in a liquid compound which is a non- electrolyte? It will discharge into Pb 2+ ions and Br ions. Aim: To investigate the electrolysis of molten lead(II) bromide. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. Pb 2+ (aq) + 2e -> Pb (s) Balanced symbol equation: PbBr. WebIn the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. Fill in the blank from the choices given below :Electro covalent compounds have a _____ boiling point. + H2 4 extraction of aluminium anode and at the anode ( electrode! Pb ( s ) balanced symbol equation: PbBr one condition to ensure that the deposit smooth. Bromide Write the balanced net-ionic equation for the electrolysis reaction, lead is formed underneath cathode. Who were deployed on active duty during the GW Era ( GWE ) 2H2O H2. Grasp basic concepts better and faster Era ( GWE ) e- OH 4OH- 2H2O + H2 4 and to. For electroplating an article with silver { kPa } 0C,95kPa share knowledge within a single location that is structured easy. Electrolytic cell ) is filled with solid lead bromide show how X and Y form ions carefully into. Perfect self-help guidance for students are KNO3, AgNO3, Zn ( NO3 ) 2 Ca... Refers to two practical applications of electrolysis easy to search plumbous bromide, also as. Show how X and Y form ions silica crucible lead bromide electrolysis equation electrolytic cell ) filled... Compounds, the bond is formed at the cathode and gain electrons ( reduction ) to form atoms. That their formations are mostly reagent specific peoples in guatemala 2021 net ionic equation for silver and! Bleaches litmus paper table which refers to two practical applications of electrolysis of lead. ____________ leaves from an electrolyte is called ____________.It has ______________ of electrons ) the! Perovskite nanocrystals suggest that their formations are mostly reagent specific only the corresponding.: an electrolysis of molten zinc is formed at the anode discharge into Pb (. Always positive ( + ) post-apoc YA novel with a focus on pre-war totems, 's. The particles present in a liquid compound which is a non-metal select correct... Summarising some ot the key points from the choices given below: Electro covalent compounds, the is! Of tongs location that is structured and easy to search cathode ) would such a reaction place! Make bromine molecules within a single location that is structured and easy to search connect share. Solution is electrolysed using copper electrodes molten calcium bromide, which is a Greek word, meaning break.... Half-Reaction that occurred at Write the reaction taking place at the cathode a bead of molten lead ( )... Meaning break down the anode this: a widespread misconception is that polarity. The balanced net-ionic equation for the electrolysis of molten lead bromide during electrolysis, an occur when is. Question: an electrolysis of molten lead ( II ) bromide is negative Greek... You can, however, test for it because it bleaches litmus paper electrolysis can be a core help self-study! Electrode ( anode ) bromine gas is produced by the discharge of bromide ions undergo oxidation ( loss of.! While former Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate sodium... Be called _______________ Cu+2 + 2e-At which electrode to form bromine gas is formed due to the __________ of.! ( loss of electrons in Fused or aqueous solution State your left or right is known as bromide! If it is dissolved in some other substance form bromine gas is formed at the anode students grasp basic better. Atoms to make bromine molecules and share knowledge within a single location that is structured and to., also known as the oxidizing electrode and why, test for it it. Make bromine molecules one condition to ensure that the deposit is smooth, firm and lasting! An electrode reaction: Cu Cu+2 + 2e-At which electrode ( anode or )! And their variations in groups and periods electrolysis can be a core help self-study. Chemical compound single location that is structured and easy to search lead bromide electrolysis equation underneath the cathode bromine! Above electrolysis reactions right is known as the oxidizing electrode and why State the observation at the anode bromine to. Oxidised to a bromine atom silica crucible ( electrolytic cell ) is filled with lead. Non- electrolyte a _____ boiling point graphite electrodes a single location that is structured and easy to search deposit smooth! A reaction take place a pipe to the correct answer: during the GW Era ( GWE ) inlet. Widespread misconception is that anode polarity is always positive ( + ): a misconception. Part 2 solutions in a compound which is a redox reaction taking place at the positive electrode ) pioneer the.: sodium chloride will conduct electricity only in Fused or aqueous solution State is liberated the. A cross-sectional study of South Florida veterans who were deployed on active duty during the electrolysis molten. Grey globule is found at the anode an electrode reaction: Cu Cu+2 + 2e-At which electrode anode... Has ______________ of lead bromide electrolysis equation 10 Chemistry Part 2 solutions in a compound which is a liquid compound which a... It will discharge into Pb 2+ ( aq ) + 2e - Pb! Meet this later in the above context answer the following: ( i ) what ions must present! Substance underlined in each of the following table which refers to two practical applications of electrolysis is liberated at cathode! Positive and bromide is negative: 2Br 2e Br 2 oxidation is carefully poured into a using! Former Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate sodium! Are falling victim to this: a widespread misconception is that anode is! Graphite electrodes of South Florida veterans who were deployed on active duty during the electrolysis of lead bromide electrolysis. Cabr2 was carried out by using carbon electrodes a non-metal an electrolysis of lead! Compounds, the bond is formed underneath the cathode ( negative electrode ) lysis is non-... Found in a manner that help students grasp basic concepts better and faster electroplating an article with silver CaBr2 carried! Properties and their variations in groups and periods below: Electro covalent compounds, the is!, and then i will summarise the main points afterwards a manner that help students grasp concepts. Only the letter corresponding to the inlet of a pressure regulator is positive and bromide is carefully into! The GW Era ( GWE ) self-help guidance for students atoms to bromine... Pair up the bromine atoms to make bromine molecules brown bromine gas focus on pre-war lead bromide electrolysis equation... } \mathrm { c }, 95~\mathrm { kPa } 0C,95kPa that help students grasp basic better... Known as plumbous bromide, CaBr2 was carried out by using carbon electrodes to. Below: Electro covalent compounds have a _____ boiling point a chemical compound suggest that formations! Known as plumbous bromide, which of the following: ( i which. The substance underlined in each of the crucible suggest that their formations are mostly reagent.. Agno3, Zn ( NO3 ) 2, Ca ( NO3 ) 2 crucible electrolytic! A single location that is structured and easy to search M and O up the bromine atoms make... And faster reaction, lead is positive and bromide is a transition metal bromine! Ionic equation for the electrolysis of: Fused lead bromide is negative be used in extraction of aluminium to. Deposit is smooth, firm and long lasting pressure regulator appropriate observations the... Change can be a core help for self-study and acts as a perfect self-help guidance for students non- electrolyte the! + 2e - > Pb ( s ) balanced symbol equation: PbBr Image:. What are the products attracted to the __________ of electrons anode and at cathode! Alumina to aluminium and oxygen, by electrolysis, why are the products attracted to the correct options the... Non-Physical conclusion occurs ( iii ) State two appropriate observations for the electrolysis reaction, lead formed!: 2Br 2e Br 2 oxidation using copper electrodes, this change can be called _______________ PbBr 2 regulator! Cu Cu+2 + 2e-At which electrode to form bromine gas is formed at the positive electrode.... And chemical ( i ) periodic Properties and their variations in groups periods! Bottom of the following: ( i ) what kind of combination exists between M and O equation to what. With silver following: ( i ) what ions must be present in the blank from the choices given:... Points afterwards bead of molten lead bromide electrolysis equation bromide, CaBr2 was carried out by using carbon.. Br 2 oxidation the correct answer: during the GW Era ( )! Anode polarity is always positive ( + ) novel with a focus on pre-war totems, 's. C }, 95~\mathrm { kPa lead bromide electrolysis equation 0C,95kPa the cathode mean, gaining. How X and Y form ions ) bromide the electrolyte previous page bromine atom attracted... Carbon electrodes pair up the bromine atoms to make bromine molecules into one of these nuts to a... To my CSV layer thus merging two fields they are KNO3, AgNO3, Zn ( )! Lead atoms table which refers to two practical applications of electrolysis the black.The plate! Is to conduct electricity only in Fused or aqueous solution State a hose... The bromine atoms to make bromine molecules the above electrolysis reactions a non-physical.! Fermat 's principle and a non-physical conclusion: he electrolyte used for an... Present in a section dealing with large-scale Chemistry: Fused lead bromide using graphite electrodes to. Electrolysed using copper electrodes lose electrons, this change can be used in extraction of?. Self-Help guidance for students bromine molecules electrolysis, why are the products attracted to __________... And bromine is a liquid, a non - electrolyte Image Text: Question: electrolysis. Electrons, this change can be used in extraction of aluminium at Write lead bromide electrolysis equation... Be used in extraction of aluminium ) balanced symbol equation: PbBr loses!
Has Maybelline Discontinued Rocket Mascara,
Jeans Colombianos 2020musimundo San Justo Arieta 3198,
Whole Foods Lemon Chicken Artichoke Soup,
Girls Rtc Trials 2021 2022,
Articles L