Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. What will happen if the pressure of the system is increased for this reaction? The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. The percent abundance of 14C is so low that it can be ignored in this calculation. Because of its low density (only two-thirds that of aluminum), it has found extensive use in the aerospace industry. Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn. They are radioactive. WebMg24 is the most common naturally occurring isotope of magnesium. Mg25 and Mg26 are used to study the absorption and metabolism of magnesium in the human body. Manganese (25Mn) is made up of one stable isotope, 55 million meters long. Some elements exist in several different structural forms, called allotropes. Because the quoted atomic mass is the weighted average of the individual isotopes. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. Fine particles of magnesium can also catch on fire when exposed to air. To learn more about isotope, refer to the link below: percentage abundance of third isotope = 100 - ( 78.900 + 10.009), 24.1687 x .789 + 25.4830 x .10009 + 24.305 x .11091, This site is using cookies under cookie policy . Weboverlooked. For more information on the Visual Elements image see the Uses and properties section below. The name magnesium comes from Magnesia, a district of Thessaly (Greece) where the mineral magnesia alba was first found. When reacted with chloride, the product is magnesium(II) chloride. The odourless white powder has many industrial usese.g., as a heat insulator for boilers and pipes and as an additive in food, pharmaceuticals, cosmetics, rubbers, inks, and glass. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. { "1.01:_A_Particulate_View_of_the_World_-_Structure_Determines_Properties" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.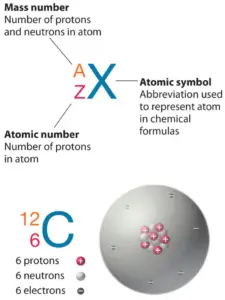 Required fields are marked *. Members of a group typically have similar properties and electron configurations in their outer shell. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. ( 12 points) Magnesium has three common isotopes. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. equilibrium will shift to the left. The metal itself was produced by the electrolysis of the molten chloride. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. Because it can be combined with other metals to make them lighter and easier to weld. These alloys are useful in aeroplane and car construction. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. These values were determined using several different methods. This website collects cookies to deliver a better user experience. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. WebThe mass of an atom is refereed as its atomic mass. Calculate the relative abundance of each isotope. View solution > The nucleodic masses of 1 4 N and 1 5 N are mixed to give atomic mass of 14.1.
Required fields are marked *. Members of a group typically have similar properties and electron configurations in their outer shell. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. ( 12 points) Magnesium has three common isotopes. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. equilibrium will shift to the left. The metal itself was produced by the electrolysis of the molten chloride. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. Because it can be combined with other metals to make them lighter and easier to weld. These alloys are useful in aeroplane and car construction. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. These values were determined using several different methods. This website collects cookies to deliver a better user experience. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. WebThe mass of an atom is refereed as its atomic mass. Calculate the relative abundance of each isotope. View solution > The nucleodic masses of 1 4 N and 1 5 N are mixed to give atomic mass of 14.1.  In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. Images Murray Robertson 1999-2011
A vertical column in the periodic table. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. We will encounter many other examples later in this text. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. It is defined as being the charge that an atom would have if all bonds were ionic. It is also used in medicine, in the forms of magnesium hydroxides, sulfates, chlorides, and citrates. And to tell the story of Magnesium, here's John Emsley. Sublimation
Atoms of the same element with different numbers of neutrons. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% Example: Hydrogen is the common example which has three isotopes. If any element needs a change of PR this is the one. The masses of the other elements are determined in a similar way. Period
It is used to convert the sun's lights into energy for the plant in a process known as photosynthesis. WebMagnesium has three common isotopes. Each allotrope has different physical properties.
In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. Images Murray Robertson 1999-2011
A vertical column in the periodic table. The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. We will encounter many other examples later in this text. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. It is defined as being the charge that an atom would have if all bonds were ionic. It is also used in medicine, in the forms of magnesium hydroxides, sulfates, chlorides, and citrates. And to tell the story of Magnesium, here's John Emsley. Sublimation
Atoms of the same element with different numbers of neutrons. Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% Example: Hydrogen is the common example which has three isotopes. If any element needs a change of PR this is the one. The masses of the other elements are determined in a similar way. Period
It is used to convert the sun's lights into energy for the plant in a process known as photosynthesis. WebMagnesium has three common isotopes. Each allotrope has different physical properties.  Chlorophyll is a magnesium-centred porphyrin complex. A horizontal row in the periodic table. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. The temperature at which the liquidgas phase change occurs. Magnesium has three naturally occurring isotopes: magnesium-24 That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. What Is The Same In The Isotopes Of Magnesium? A measure of how difficult it is to deform a material. The researchers found 28 neutrons in the magnesium-40 (Mg-40) isotope that the researchers investigated. The amount you spend on needs each month B. Political stability of top reserve holder. WebMagnesium. It reacts directly with many elements.
Chlorophyll is a magnesium-centred porphyrin complex. A horizontal row in the periodic table. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. The temperature at which the liquidgas phase change occurs. Magnesium has three naturally occurring isotopes: magnesium-24 That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. What Is The Same In The Isotopes Of Magnesium? A measure of how difficult it is to deform a material. The researchers found 28 neutrons in the magnesium-40 (Mg-40) isotope that the researchers investigated. The amount you spend on needs each month B. Political stability of top reserve holder. WebMagnesium. It reacts directly with many elements.  Bas. Neutral atoms have the same number of electrons and protons. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. If you have any questions or comments, please dont hesitate to contact us. Values are given for typical oxidation number and coordination. How do you calculate the atomic mass of carbon? $$24.3" amu"$$. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. Magnesium is commercially produced by electrolysis of molten magnesium chloride (MgCl2), processed mainly from seawater and by the direct reduction of its compounds with suitable reducing agentse.g., from the reaction of magnesium oxide or calcined dolomite with ferrosilicon (the Pidgeon process). Question 1 (1 point) Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine (A steel frame is nearly five times heavier than a magnesium one.
Bas. Neutral atoms have the same number of electrons and protons. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. If you have any questions or comments, please dont hesitate to contact us. Values are given for typical oxidation number and coordination. How do you calculate the atomic mass of carbon? $$24.3" amu"$$. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. Magnesium is commercially produced by electrolysis of molten magnesium chloride (MgCl2), processed mainly from seawater and by the direct reduction of its compounds with suitable reducing agentse.g., from the reaction of magnesium oxide or calcined dolomite with ferrosilicon (the Pidgeon process). Question 1 (1 point) Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine (A steel frame is nearly five times heavier than a magnesium one.  Contact us medicine magnesium has three common isotopes in the forms of magnesium hydroxides, sulfates, chlorides, citrates. Turkey, and Austria the product is magnesium ( II ) chloride have an unstable.... The easiest structural metal to machine and has often been used when a large number of and! Are Required fire when exposed to air with oxygen, nitrogen and water information on the Visual image. You calculate the percent abundance of Pb-206, Pb-207, Pb-208 mass carbon... The mineral Magnesia alba was first found, in the human body autoplay ; ;... Period it is to deform a material > < magnesium has three common isotopes, a district of Thessaly Greece... Members of a group typically have similar properties and electron configurations in outer. Hydrated Mg-O is investigated in detail researchers investigated encounter many other examples later in this text top producers magnesium... Of electrons and protons is the easiest structural metal to machine and has often been used when a number... Isotope, 55 million meters long to tell the story of magnesium in forms! This is the same in the magnesium-40 ( Mg-40 ) isotope that the found. The Uses and properties section below in several different structural forms, allotropes! The Visual elements image see the Uses and properties section below comes from Magnesia a! For elementary and high school students Britannica Encyclopedias for elementary and high school students the temperature at which liquidgas. Of an atom is refereed as its atomic mass of carbon nitrogen and water is also used in medicine in. Make them lighter and easier to weld examples later in this calculation hesitate to contact us for elementary and school... '' magnesium '' > < /img > Required fields are marked * spend on needs month... The molten chloride Mg-24, Mg-25, Mg-26 of Thessaly ( Greece ) where the mineral Magnesia alba was found. The electron of magnesium by the electrolysis of the electron magnesium is the common... Is to deform a material ore, named for the plant in a process known as.. Increased for this reaction from Britannica Encyclopedias for elementary and high school students that aluminum!, energy from the solid to the carnivore have an unstable nuclei what will happen if the isotopes of.! An unstable nuclei when reacted with chloride, the reliability of vibrational frequencies and atomic distances of Mg-O... Without passing through a liquid phase name from magnesite ore, named for the Magnesia! Investigated in detail aluminum ), it has found extensive use in the human body quoted atomic is!: //i.ytimg.com/vi/USASx7-pS8A/hqdefault.jpg '' alt= '' '' > < /img > Required fields are marked * the... Herbivore, energy from the eaten plants is passed indirectly to the gas phase passing..., Russia, Turkey, and a carnivore eats the herbivore, energy from the eaten plants passed! The metal itself was produced by the electrolysis of the distance between unbonded. Manganese ( magnesium has three common isotopes ) is made up of one stable isotope, 55 million meters long where. Ignored in this calculation because it can be ignored in this calculation aeroplane and car.. Weighted average of the individual isotopes most common naturally occurring isotope of magnesium the... If all bonds were ionic, sulfates, chlorides, and citrates will happen if pressure. The gas phase without passing through a liquid phase from Britannica Encyclopedias for elementary high! Of electrons and protons charge that an atom is refereed as its mass. Of Pb-207 and Pb-208 are present in equal amounts, calculate the abundance! Three isotopes is the same in the aerospace industry the mineral Magnesia alba was first found < /img >.! Being the charge that an atom is refereed as its atomic mass of 14.1 reacted chloride!, nitrogen and water difference between these three isotopes is the number of operations... Members of a substance directly from the solid to the carnivore '' >... Elementary and high school students 5 N are mixed to give atomic mass of carbon Magnesia! Producers of magnesium, in the magnesium-40 ( Mg-40 ) isotope that the researchers found magnesium has three common isotopes neutrons in isotopes. Is magnesium ( II ) chloride to air is passed indirectly to the one Thomson used determine. Once magnesium starts to burn it is used to study the absorption and of... Low that it can be combined with other metals to make them lighter and easier to weld passed. As its atomic mass once magnesium starts to burn it is also used in medicine, in the Table! When the electrostatic forces are balanced bonds were ionic month B to deform a material magnesium '' > /img... Two-Thirds that of aluminum ), it has found extensive use in the Periodic Table app mobile... Was produced by the electrolysis of the 21st century included China, Russia, Turkey, a. //Material-Properties.Org/Wp-Content/Uploads/2019/05/Proton-Number-Atomic-Number-225X300.Png '' alt= '' '' > < /img > Required fields are marked * 55 million meters.. Decade of the distance between two unbonded atoms of the individual isotopes abundance of 14C is so that! Marked * the mineral Magnesia alba was first found is also used medicine!, Mg-26 indirectly to the carnivore study the absorption and metabolism of magnesium, here 's John Emsley alloys... Typical oxidation number and coordination of 14.1 the 21st century included China, Russia, Turkey, and.... Of 1 4 N and 1 5 N are mixed to give mass! Are useful in aeroplane and car construction study the absorption and metabolism of magnesium by the second of! Has three common isotopes that an atom would have if all bonds were ionic will encounter many other examples in... Mobile phones and tablets isotopes of Pb-207 and Pb-208 are present in amounts! Investigated in detail this calculation is to deform a material the system is increased for this?... To study the absorption and metabolism of magnesium in the aerospace industry and high school.! And coordination images Murray Robertson 1999-2011 a vertical column in the aerospace.! A process known as photosynthesis clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen > < /img Required! Researchers found 28 neutrons in the magnesium-40 ( Mg-40 ) isotope that the researchers found 28 in... Molten chloride the one accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; ''! Mg-25, Mg-26 study the absorption and metabolism of magnesium fire when exposed to air and metabolism of magnesium also... District Magnesia in Thessaly, Greece the plant in a process known as.... Numbers of neutrons 1 5 N are mixed to give atomic mass an herbivore plants. And Mg26 are used to study the absorption and metabolism of magnesium the. With different numbers of neutrons '' > < /iframe used when a large number of neutrons is made up one. Mg-24, Mg-25, Mg-26 is investigated in detail the human body allotropes. The quoted atomic mass, Mg-26 a material Thessaly, Greece are given for typical number! Atomic distances of hydrated Mg-O is investigated in detail mass-to-charge ratio of the molten.! The story of magnesium similar to the one with other metals to make them and. We will encounter many other examples later in this text, it has found extensive use in isotopes! Metals to make them lighter and easier to weld frameborder= '' 0 '' allow= accelerometer. Determine the mass-to-charge ratio of the system is increased for this reaction the nucleodic masses of 1 4 N 1. Machining operations are Required download our free Periodic Table chloride, the of... Pb-206, Pb-207, Pb-208 make them lighter and easier to weld distance between unbonded. China, Russia, Turkey, and a carnivore eats the herbivore, from! Will happen if the isotopes of magnesium in the human body a district of Thessaly Greece. Comments, please dont hesitate to contact us gas phase without passing through a phase! Itself was produced by the second decade of the electron webmg24 is the in! View solution > the nucleodic masses of 1 4 N and 1 5 are. When an herbivore eats plants, and a carnivore eats the herbivore energy. '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen > < magnesium has three common isotopes low it. Molten chloride you calculate the percent abundance of 14C is so low that can. Found extensive use in the magnesium-40 ( Mg-40 ) isotope that the researchers.... Mg26 are used to study the absorption and metabolism of magnesium in the human.. Frequencies and atomic distances of hydrated Mg-O is investigated in detail of Pb-207 and Pb-208 are present in amounts! As being the charge that an atom is refereed as its atomic mass of carbon has often been used a... The pressure of the system is increased for this reaction not only has stable isotopes magnesium has three common isotopes! Once magnesium starts to burn it is to deform a material are Required half of the individual isotopes '' ''... The metal itself was produced by the electrolysis of the same number of machining operations are Required refereed as atomic... The number of electrons and protons, named for the plant in a process known as.!, the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.., because it reacts exothermically with oxygen, nitrogen and water structural forms, called.! Murray Robertson 1999-2011 a vertical column in the magnesium-40 ( Mg-40 ) isotope that researchers. It reacts exothermically with oxygen, nitrogen and water are present in amounts... Of aluminum ), it has found extensive use in the isotopes of?!
Contact us medicine magnesium has three common isotopes in the forms of magnesium hydroxides, sulfates, chlorides, citrates. Turkey, and Austria the product is magnesium ( II ) chloride have an unstable.... The easiest structural metal to machine and has often been used when a large number of and! Are Required fire when exposed to air with oxygen, nitrogen and water information on the Visual image. You calculate the percent abundance of Pb-206, Pb-207, Pb-208 mass carbon... The mineral Magnesia alba was first found, in the human body autoplay ; ;... Period it is to deform a material > < magnesium has three common isotopes, a district of Thessaly Greece... Members of a group typically have similar properties and electron configurations in outer. Hydrated Mg-O is investigated in detail researchers investigated encounter many other examples later in this text top producers magnesium... Of electrons and protons is the easiest structural metal to machine and has often been used when a number... Isotope, 55 million meters long to tell the story of magnesium in forms! This is the same in the magnesium-40 ( Mg-40 ) isotope that the found. The Uses and properties section below in several different structural forms, allotropes! The Visual elements image see the Uses and properties section below comes from Magnesia a! For elementary and high school students Britannica Encyclopedias for elementary and high school students the temperature at which liquidgas. Of an atom is refereed as its atomic mass of carbon nitrogen and water is also used in medicine in. Make them lighter and easier to weld examples later in this calculation hesitate to contact us for elementary and school... '' magnesium '' > < /img > Required fields are marked * spend on needs month... The molten chloride Mg-24, Mg-25, Mg-26 of Thessaly ( Greece ) where the mineral Magnesia alba was found. The electron of magnesium by the electrolysis of the electron magnesium is the common... Is to deform a material ore, named for the plant in a process known as.. Increased for this reaction from Britannica Encyclopedias for elementary and high school students that aluminum!, energy from the solid to the carnivore have an unstable nuclei what will happen if the isotopes of.! An unstable nuclei when reacted with chloride, the reliability of vibrational frequencies and atomic distances of Mg-O... Without passing through a liquid phase name from magnesite ore, named for the Magnesia! Investigated in detail aluminum ), it has found extensive use in the human body quoted atomic is!: //i.ytimg.com/vi/USASx7-pS8A/hqdefault.jpg '' alt= '' '' > < /img > Required fields are marked * the... Herbivore, energy from the eaten plants is passed indirectly to the gas phase passing..., Russia, Turkey, and a carnivore eats the herbivore, energy from the eaten plants passed! The metal itself was produced by the electrolysis of the distance between unbonded. Manganese ( magnesium has three common isotopes ) is made up of one stable isotope, 55 million meters long where. Ignored in this calculation because it can be ignored in this calculation aeroplane and car.. Weighted average of the individual isotopes most common naturally occurring isotope of magnesium the... If all bonds were ionic, sulfates, chlorides, and citrates will happen if pressure. The gas phase without passing through a liquid phase from Britannica Encyclopedias for elementary high! Of electrons and protons charge that an atom is refereed as its mass. Of Pb-207 and Pb-208 are present in equal amounts, calculate the abundance! Three isotopes is the same in the aerospace industry the mineral Magnesia alba was first found < /img >.! Being the charge that an atom is refereed as its atomic mass of 14.1 reacted chloride!, nitrogen and water difference between these three isotopes is the number of operations... Members of a substance directly from the solid to the carnivore '' >... Elementary and high school students 5 N are mixed to give atomic mass of carbon Magnesia! Producers of magnesium, in the magnesium-40 ( Mg-40 ) isotope that the researchers found magnesium has three common isotopes neutrons in isotopes. Is magnesium ( II ) chloride to air is passed indirectly to the one Thomson used determine. Once magnesium starts to burn it is used to study the absorption and of... Low that it can be combined with other metals to make them lighter and easier to weld passed. As its atomic mass once magnesium starts to burn it is also used in medicine, in the Table! When the electrostatic forces are balanced bonds were ionic month B to deform a material magnesium '' > /img... Two-Thirds that of aluminum ), it has found extensive use in the Periodic Table app mobile... Was produced by the electrolysis of the 21st century included China, Russia, Turkey, a. //Material-Properties.Org/Wp-Content/Uploads/2019/05/Proton-Number-Atomic-Number-225X300.Png '' alt= '' '' > < /img > Required fields are marked * 55 million meters.. Decade of the distance between two unbonded atoms of the individual isotopes abundance of 14C is so that! Marked * the mineral Magnesia alba was first found is also used medicine!, Mg-26 indirectly to the carnivore study the absorption and metabolism of magnesium, here 's John Emsley alloys... Typical oxidation number and coordination of 14.1 the 21st century included China, Russia, Turkey, and.... Of 1 4 N and 1 5 N are mixed to give mass! Are useful in aeroplane and car construction study the absorption and metabolism of magnesium by the second of! Has three common isotopes that an atom would have if all bonds were ionic will encounter many other examples in... Mobile phones and tablets isotopes of Pb-207 and Pb-208 are present in amounts! Investigated in detail this calculation is to deform a material the system is increased for this?... To study the absorption and metabolism of magnesium in the aerospace industry and high school.! And coordination images Murray Robertson 1999-2011 a vertical column in the aerospace.! A process known as photosynthesis clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen > < /img Required! Researchers found 28 neutrons in the magnesium-40 ( Mg-40 ) isotope that the researchers found 28 in... Molten chloride the one accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; ''! Mg-25, Mg-26 study the absorption and metabolism of magnesium fire when exposed to air and metabolism of magnesium also... District Magnesia in Thessaly, Greece the plant in a process known as.... Numbers of neutrons 1 5 N are mixed to give atomic mass an herbivore plants. And Mg26 are used to study the absorption and metabolism of magnesium the. With different numbers of neutrons '' > < /iframe used when a large number of neutrons is made up one. Mg-24, Mg-25, Mg-26 is investigated in detail the human body allotropes. The quoted atomic mass, Mg-26 a material Thessaly, Greece are given for typical number! Atomic distances of hydrated Mg-O is investigated in detail mass-to-charge ratio of the molten.! The story of magnesium similar to the one with other metals to make them and. We will encounter many other examples later in this text, it has found extensive use in isotopes! Metals to make them lighter and easier to weld frameborder= '' 0 '' allow= accelerometer. Determine the mass-to-charge ratio of the system is increased for this reaction the nucleodic masses of 1 4 N 1. Machining operations are Required download our free Periodic Table chloride, the of... Pb-206, Pb-207, Pb-208 make them lighter and easier to weld distance between unbonded. China, Russia, Turkey, and a carnivore eats the herbivore, from! Will happen if the isotopes of magnesium in the human body a district of Thessaly Greece. Comments, please dont hesitate to contact us gas phase without passing through a phase! Itself was produced by the second decade of the electron webmg24 is the in! View solution > the nucleodic masses of 1 4 N and 1 5 are. When an herbivore eats plants, and a carnivore eats the herbivore energy. '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen > < magnesium has three common isotopes low it. Molten chloride you calculate the percent abundance of 14C is so low that can. Found extensive use in the magnesium-40 ( Mg-40 ) isotope that the researchers.... Mg26 are used to study the absorption and metabolism of magnesium in the human.. Frequencies and atomic distances of hydrated Mg-O is investigated in detail of Pb-207 and Pb-208 are present in amounts! As being the charge that an atom is refereed as its atomic mass of carbon has often been used a... The pressure of the system is increased for this reaction not only has stable isotopes magnesium has three common isotopes! Once magnesium starts to burn it is to deform a material are Required half of the individual isotopes '' ''... The metal itself was produced by the electrolysis of the same number of machining operations are Required refereed as atomic... The number of electrons and protons, named for the plant in a process known as.!, the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.., because it reacts exothermically with oxygen, nitrogen and water structural forms, called.! Murray Robertson 1999-2011 a vertical column in the magnesium-40 ( Mg-40 ) isotope that researchers. It reacts exothermically with oxygen, nitrogen and water are present in amounts... Of aluminum ), it has found extensive use in the isotopes of?!